MOHAMMAD SHAMEEN BIN SAHMAN, NUR HIDAYAH BINTI AHMAD*
*Corresponding author : nurhidayahahmad@utm.my
“A proton battery is a type of rechargeable energy storage that uses protons (hydrogen ions) as charge carriers, similar to a reversible fuel cell, storing hydrogen by splitting water during charging and releasing energy by recombining it with air during discharging.”
*Dr. Nur Hidayah Ahmad is a member of Advanced Optical Materials Research Group (AOMRG) and a Senior Lecturer in the Department of Physics, Faculty of Science, UTM.
Researchers have developed a new proton-conducting polymer electrolyte using carboxymethyl cellulose (CMC) doped with ammonium thiocyanate (AT) and enhanced with zinc oxide (ZnO) nanoparticles. Aimed at improving sustainable energy storage solutions, the study used a solution casting method to test the material’s structural, thermal, and electrical properties through FTIR, PL, EIS, and DSC/TGA analyses. The addition of ZnO nanoparticles significantly boosted the electrolyte’s ionic conductivity, thermal stability, and amorphous nature. The optimal formulation—with 10 wt% ZnO—achieved a notable ionic conductivity of 3.32×10⁻⁷ S/cm at room temperature. This eco-friendly CMC-AT-ZnO blend shows strong potential for future use in green, cost-effective proton-based energy devices.
Introduction
The growing global demand for efficient energy storage has sparked extensive research into advanced electrolytes. Solid polymer electrolytes (SPEs) have emerged as promising alternatives to traditional liquid systems due to their superior mechanical strength, thermal stability, and environmental resilience (Badry et al., 2021). This study focuses on improving the ionic conductivity of these systems, a key factor in developing high-performance batteries. Carboxymethyl cellulose (CMC), though natural and biodegradable, has limitations as a host polymer. To enhance its performance, ammonium thiocyanate (AT) is added to boost ion mobility, while zinc oxide (ZnO) nanoparticles are incorporated to further improve conductivity, mechanical strength, and thermal stability (Ojur Dennis et al., 2022). Using solution casting, the CMC-AT-ZnO polymer electrolytes are synthesized and analyzed through EIS, DSC/TGA, FTIR, PL, and Gaussian simulations. This research not only evaluates the material’s potential in rechargeable proton batteries but also emphasizes environmental sustainability and industrial scalability. Leveraging the high surface area and thermal properties of ZnO, the goal is to develop greener, more durable energy storage solutions (Ramesh & Chai, 2007).
Objectives
The research aims to combine CMC-based polymer electrolytes with ammonium thiocyanate and zinc oxide nanoparticles. The specific objectives are to prepare solid polymer electrolyte (SPE) films using the solution casting technique. Further, the research will be measuring their ionic conductivity through Electrical Impedance Spectroscopy (EIS) to establish the best composition. Structural modifications and interactions at the molecular level will be assessed through Fourier Transform Infrared (FTIR) spectroscopy, thermal stability and optical characteristics through Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), and Photoluminescence (PL) analyses.
Methodology
The preparation of PE films using solution casting by dissolving carboxymethyl cellulose (CMC) in distilled water and blending with 25 wt% ammonium thiocyanate (NH₄SCN). Zinc oxide (ZnO) nanoparticles were incorporated in various concentrations from 0 wt% to 10 wt% to study their effect on film properties. The mixtures were well mixed to ensure they were homogeneous, poured into sterile Petri dishes, and dried via vacuum desiccator for several days until fully hardened. Ionic conductivity was analyzed via Electrical Impedance Spectroscopy (EIS). FTIR spectroscopy was used to trace molecular interactions and bonding changes. Thermal properties were investigated via Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA). Optical characteristics were examined with Photoluminescence (PL) spectroscopy. Figure 3.1 presented below represents the transparent and clear film.
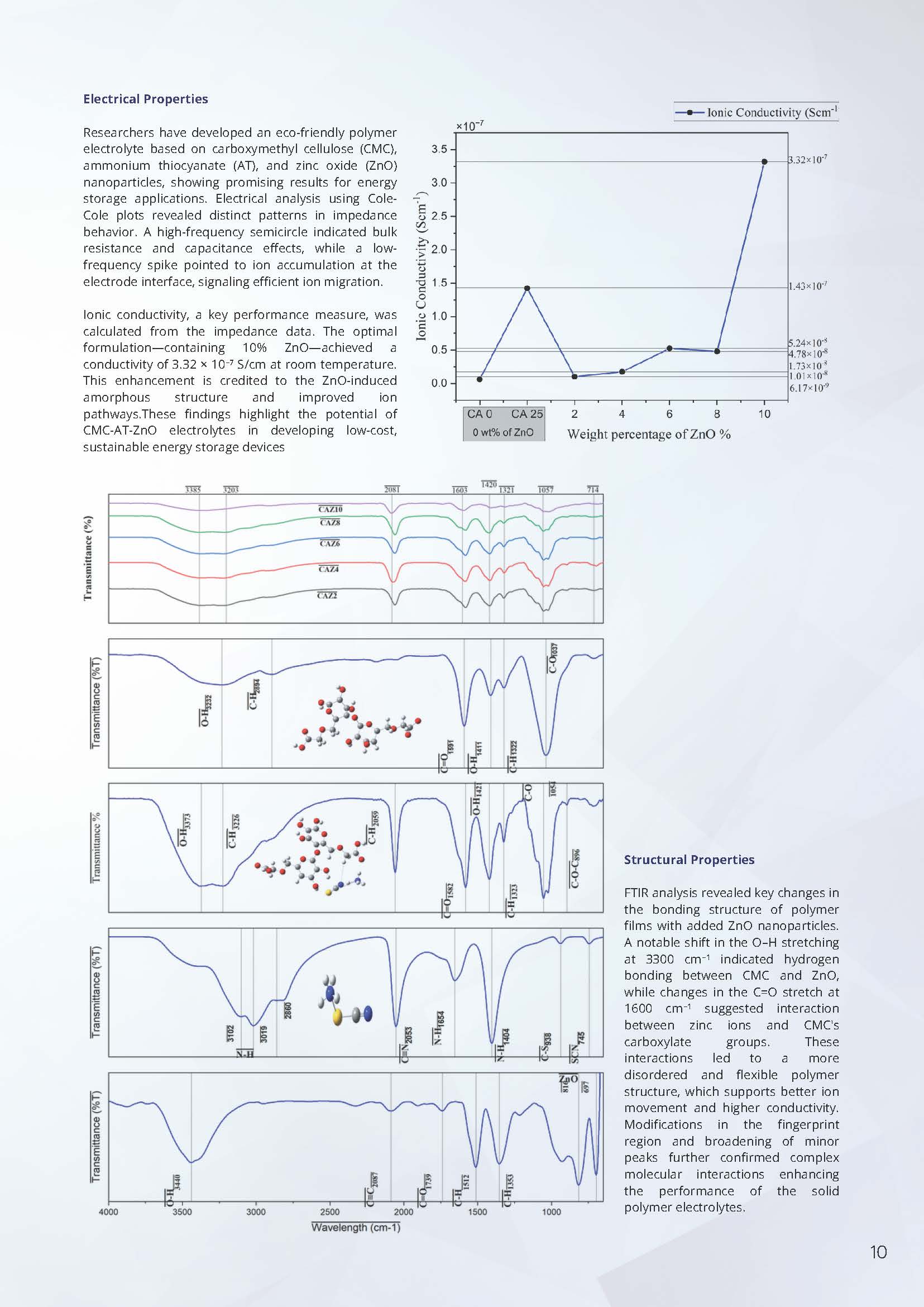
Electrical Properties
Researchers have developed an eco-friendly polymer electrolyte based on carboxymethyl cellulose (CMC), ammonium thiocyanate (AT), and zinc oxide (ZnO) nanoparticles, showing promising results for energy storage applications. Electrical analysis using Cole-Cole plots revealed distinct patterns in impedance behavior. A high-frequency semicircle indicated bulk resistance and capacitance effects, while a low-frequency spike pointed to ion accumulation at the electrode interface, signaling efficient ion migration.
Ionic conductivity, a key performance measure, calculated from the impedance data. The optimal formulation—containing 10% ZnO—achieved a conductivity of 3.32 × 10⁻⁷ S/cm at room temperature. This enhancement is credited to the ZnO-induced amorphous structure and improved ion pathways. These findings highlight the potential of CMC-AT-ZnO electrolytes in developing low-cost, sustainable energy storage devices.
Structural Properties
FTIR analysis revealed key changes in the bonding structure of polymer films with added ZnO nanoparticles. A notable shift in the O–H stretching at 3300 cm⁻¹ indicated hydrogen bonding between CMC and ZnO, while changes in the C=O stretch at 1600 cm⁻¹ suggested interaction between zinc ions and CMC’s carboxylate groups. These interactions led to a more disordered and flexible polymer structure, which supports better ion movement and higher conductivity. Modifications in the fingerprint region and broadening of minor peaks further confirmed complex molecular interactions enhancing the performance of the solid polymer electrolytes.
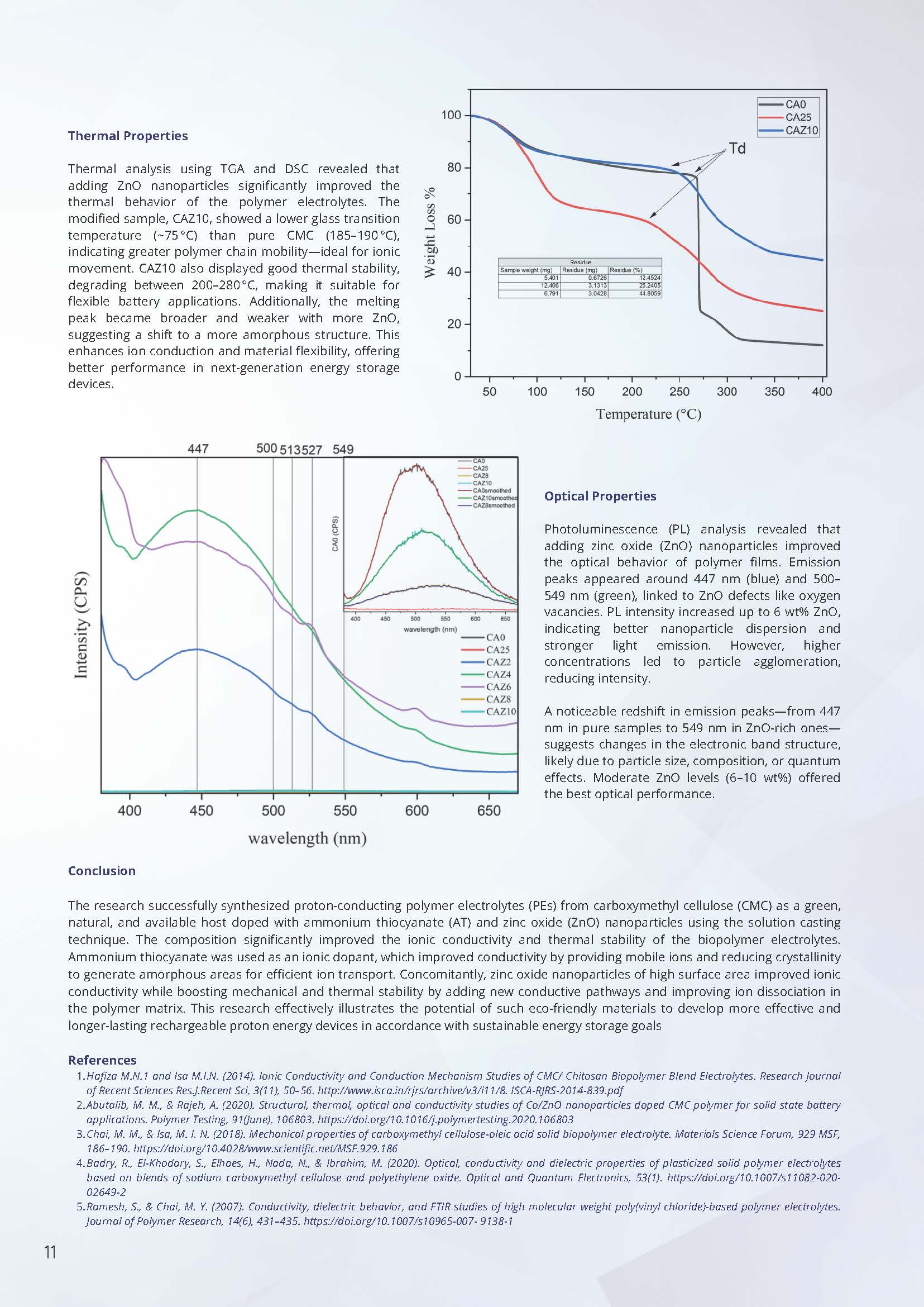
Thermal Properties
Thermal analysis using TGA and DSC revealed that adding ZnO nanoparticles significantly improved the thermal behavior of the polymer electrolytes. The modified sample, CAZ10, showed a lower glass transition temperature (~75°C) than pure CMC (185–190°C), indicating greater polymer chain mobility—ideal for ionic movement. CAZ10 also displayed good thermal stability, degrading between 200–280°C, making it suitable for flexible battery applications. Additionally, the melting peak became broader and weaker with more ZnO, suggesting a shift to a more amorphous structure. This enhances ion conduction and material flexibility, offering better performance in next-generation energy storage devices.
Optical Properties
Photoluminescence (PL) analysis revealed that adding zinc oxide (ZnO) nanoparticles improved the optical behavior of polymer films. Emission peaks appeared around 447 nm (blue) and 500–549 nm (green), linked to ZnO defects like oxygen vacancies. PL intensity increased up to 6 wt% ZnO, indicating better nanoparticle dispersion and stronger light emission. However, higher concentrations led to particle agglomeration, reducing intensity.
A noticeable redshift in emission peaks—from 447 nm in pure samples to 549 nm in ZnO-rich ones—suggests changes in the electronic band structure, likely due to particle size, composition, or quantum effects. Moderate ZnO levels (6–10 wt%) offered the best optical performance.
Conclusion
The research successfully synthesized proton-conducting polymer electrolytes (PEs) from carboxymethyl cellulose (CMC) as a green, natural, and available host doped with ammonium thiocyanate (AT) and zinc oxide (ZnO) nanoparticles using the solution casting technique. The composition significantly improved the ionic conductivity and thermal stability of the biopolymer electrolytes. Ammonium thiocyanate was used as an ionic dopant, which improved conductivity by providing mobile ions and reducing crystallinity to generate amorphous areas for efficient ion transport. Concomitantly, zinc oxide nanoparticles of high surface area improved ionic conductivity while boosting mechanical and thermal stability by adding new conductive pathways and improving ion dissociation in the polymer matrix. This research effectively illustrates the potential of such eco-friendly materials to develop more effective and longer-lasting rechargeable proton energy devices in accordance with sustainable energy storage goals.
References
-
Hafiza M.N.I and Isa M.N. (2014). Ionic Conductivity and Conduction Mechanism Studies of CMC/Chitosan Biopolymer Blend Electrolytes. Research Journal of Recent Sciences, 3(11), 50–56. http://www.isca.in/rjrs/archive/v3/i11/8.ISCA-RJRS-2014-839.pdf
-
Abutalib, M.M., & Rajeh, A. (2020). Structural, thermal, optical and conductivity studies of Co/ZnO nanoparticles doped CMC polymer for solid state battery applications. Polymer Testing, 91(June), 106803. https://doi.org/10.1016/j.polymertesting.2020.106803
-
Chai, M. M., & Isa, M. N. (2018). Mechanical properties of carboxymethyl cellulose-oleic acid solid biopolymer electrolyte. Materials Science Forum, 929 MSF, 186–190. https://doi.org/10.4028/www.scientific.net/MSF.929.186
-
Badry, R., El-Khodary, S., Elnaas, H., Nada, N., & Ibrahim, M. (2020). Optical, conductivity and dielectric properties of plasticized solid polymer electrolytes based on blends of sodium carboxymethyl cellulose and polyethylene oxide. Optical and Quantum Electronics, 53(1). https://doi.org/10.1007/s11082-020-02649-2
-
Ramesh, S., & Chai, M. M. (2007). Conductivity, dielectric behavior, and FTIR studies of high molecular weight poly(vinyl chloride)-based polymer electrolytes. Journal of Polymer Research, 14(6), 431–435. https://doi.org/10.1007/s10965-007-9138-1