FARHAN SHAZWAN SHAH SHABRANI, NORSHAHIRAH MOHAMAD SAIDI*, NURHAFIZAH HASIM, NUR HIDAYAH AHMAD, MUHAMAD AMIRUL AIZAT ABDUL ABDAH
*Corresponding author: norshahirah.ms@utm.my
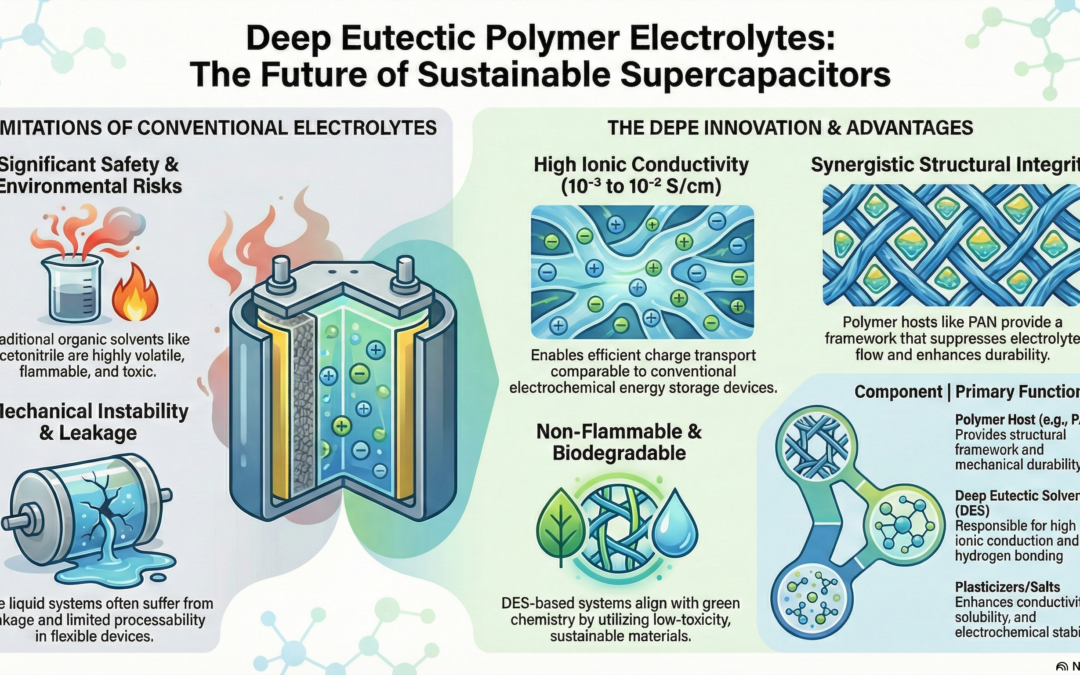
Deep eutectic solvents (DES) have emerged as a highly promising class of green ionic media for polymer electrolytes in supercapacitor applications due to their unique physicochemical properties, environmental compatibility, and tunability, which directly address the limitations associated with conventional organic and inorganic electrolytes. Formed through strong hydrogen bonding interactions between hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD), DES exhibit melting points significantly lower than those of their individual components, resulting in liquid or quasi-liquid systems at room temperature without the need for complex synthesis or purification steps. This characteristic, coupled with their negligible vapour pressure, non-flammability, and low toxicity, positions DES as a sustainable alternative to volatile organic solvents such as acetonitrile and ethanol, which are widely used in commercial supercapacitors but pose serious safety and environmental risks.
From an electrochemical perspective, DES possess relatively high ionic conductivities, typically in the range of 10⁻³ to 10⁻² Scm⁻¹, making them suitable for efficient charge transport within electrochemical energy storage devices. The extensive hydrogen bonding network inherent to DES facilitates effective ion solvation and dissociation, thereby minimizing ion pairing and enhancing the availability of mobile charge carriers. However, despite these advantages, the direct use of liquid DES in supercapacitor devices can be hindered by mechanical instability, leakage concerns, and limited processability, particularly in flexible or solid-state configurations. To overcome these challenges, DES are increasingly incorporated into polymer matrices to form deep eutectic polymer electrolytes (DEPE), which combine the electrochemical advantages of DES with the mechanical integrity and dimensional stability of polymers.
In such hybrid systems, the polymer host provides a structural framework that suppresses electrolyte flow and enhances durability, while the DES phase remains responsible for ionic conduction. Polyacrylonitrile (PAN), in particular, has been widely adopted as a host polymer due to its excellent thermal stability, chemical resistance, and ability to form uniform, flexible films, all of which are critical for practical supercapacitor assembly. The incorporation of DES into the PAN matrix disrupts the polymer’s crystalline domains, increasing the amorphous phase and facilitating greater segmental motion of polymer chains, which in turn enhances ion transport throughout the electrolyte. Additionally, the polar functional groups present in PAN interact favourably with the DES components, promoting homogeneous dispersion and stable electrolyte morphology. The performance of DES-based polymer electrolytes is further improved through the inclusion of suitable conducting salts, such as lithium perchlorate, whose small ionic radius and high dissociation capability contribute to increased ionic mobility and a wider electrochemical stability window. The perchlorate anion exhibits weak coordination with both the polymer matrix and the DES components, reducing ion aggregation and ensuring a higher concentration of free charge carriers available for conduction.
Moreover, the presence of high-dielectric-constant plasticizers, such as propylene carbonate, further enhances the ionic conductivity of DEPE systems by lowering viscosity, increasing salt solubility, and promoting polymer chain flexibility. These synergistic interactions between DES, polymer host, salt, and plasticizer result in electrolyte systems that exhibit improved ionic conductivity, mechanical flexibility, thermal stability, and electrochemical robustness compared to conventional polymer electrolytes. Importantly, the use of DES significantly enhances the sustainability profile of supercapacitors by reducing reliance on hazardous solvents and enabling the use of biodegradable or bio-derived components, aligning energy storage technology with global environmental and safety regulations.
Dr. Norshahirah Mohamad Saidi is a member of Advanced Optical Materials Research Group (AOMRG) and a Senior Lecturer in the Department of Physics, Faculty of Science, UTM.
Furthermore, DES-based polymer electrolytes demonstrate good compatibility with carbon-based electrodes commonly employed in supercapacitors, facilitating stable electrode–electrolyte interfaces that are essential for long-term cycling performance. Despite these advances, challenges remain in optimizing long-term electrochemical stability, interfacial resistance, and large-scale manufacturability, which are critical for commercial deployment. Nevertheless, ongoing research into DES composition optimization, polymer–electrolyte interactions, and electrolyte–electrode interface engineering continues to demonstrate the strong potential of DES-based polymer electrolytes as viable green alternatives for next-generation supercapacitors. Overall, the integration of deep eutectic solvents into polymer electrolyte systems represents a significant step toward achieving high-performance, safe, and environmentally benign energy storage devices, reinforcing the role of green chemistry principles in the future development of sustainable electrochemical technologies.
Acknowledgement
This research was funded by the UTM Encouragement Research (PY/2024/01390).
References
-
1,3–Propanediol. (n.d.). Retrieved October 13, 2025, from https://webbook.nist.gov/cgi/cbook.cgi?ID=C504632&type=IR-SPEC&Index=1
-
Di Pietro, M. E., & Mele, A. (2021). Deep eutectics and analogues as electrolytes in batteries. Journal of Molecular Liquids, 338, 116597. https://doi.org/10.1016/j.molliq.2021.116597
-
FTIR spectra of Polyacrylonitrile (PAN) scaffold and… | Download Scientific Diagram. (n.d.). Retrieved October 13, 2025, from https://www.researchgate.net/figure/FTIR-spectra-of-Polyacrylonitrile-PAN-scaffold-and-polyacrylonitrile-kefiran-scaffold_fig3_338932230
-
FTIR spectrums of ethylene glycol (a) and EGDM (b)… | Download Scientific Diagram. (n.d.). Retrieved October 13, 2025, from https://www.researchgate.net/figure/FTIR-spectrums-of-ethylene-glycol-a-and-EGDM-b_fig5_338868522
-
Huang, Y. C., Chu, Y. L., Chang, C. C., Liu, Y., Teng, H., Chen, B. H., & Jian, J. S. (2023). Ultraviolet-cured composite polymer electrolyte containing deep-eutectic-solvent and aluminum oxide filler for lithium-metal batteries with enhanced cycle life. Journal of Electroanalytical Chemistry, 940, 117455. https://doi.org/10.1016/J.JELECHEM.2023.117455
-
Karimi, M. B., Mohammadi, F., & Hoosini, K. (2020). Potential use of deep eutectic solvents (DESs) to enhance anhydrous proton conductivity of Nafion 115 membrane for fuel cell applications. Journal of Membrane Science, 611, 118217. https://doi.org/10.1016/J.MEMSCI.2020.118217
-
Kumar, P., Agrawal, N., Paliwal, G., Banerjee, K., Gupta, M., & Kumar, Y. (2025). Effect of ethylene glycol on deep eutectic solvent properties: dual functionality of zinc chloride as hydrogen bond acceptor and salt. Results in Chemistry, 17, 102570. https://doi.org/10.1016/J.RECHEM.2025.102570
-
Mandal, S., Mendhe, A. B., Rakhade, H. M., Barse, N. S., Roy, M., Rosaiah, P., Park, T., Lee, H. S., Mendhe, A. C., & Kim, D. (2025). Recent advancement and design in supercapacitor hybrid electrode materials: Bridging the gap between energy and power density. Chemical Engineering Journal Advances, 2, 100690. https://doi.org/10.1016/J.CEJA.2024.100690
-
Mishra, R., Malviya, M., & Tiwari, P. (2025). Revolutionizing aqueous batteries: Exploring the challenges and thriving advancements. Journal of Energy Storage, 124, 116787. https://doi.org/10.1016/J.EST.2025.116787
-
Panadian, R., Kim, D., Zhang, Y., Alfurayji, I., Prado, D. M., Maginn, E., & Burda, C. (2024). Chain length and OH-spacing effects on diol-based deep eutectic solvents. Journal of Molecular Liquids, 393, 123534. https://doi.org/10.1016/J.MOLLIQ.2023.123534
-
Sernaglia, M., Bartolomé, M., Viesca, J. L., González, R., & Battez, A. H. (2025). Application of deep eutectic solvents in lubrication: A review. Journal of Molecular Liquids, 427, 127464. https://doi.org/10.1016/J.MOLLIQ.2025.127464
-
Shi, Z., Wang, J., Guo, X., Wang, H., Nie, H., & Xue, Z. (2023). Deep eutectic solvent-assisted phase separation for polyurethane-based polymer electrolytes. Chemical Engineering Journal, 468, 143687. https://doi.org/10.1016/J.CEJ.2023.143687
-
Sim, L. N., Yahya, R., & Arof, A. K. (2016). Infrared studies of polyacrylonitrile-based polymer electrolytes incorporated with lithium bis(trifluoromethanesulfonimide) and urea as deep eutectic solvent. Optical Materials, 56, 140–144. https://doi.org/10.1016/J.OPTMAT.2016.01.007
-
Smith, E. L., Abbott, A. P., & Ryder, K. S. (2014). Deep Eutectic Solvents (DESs) and Their Applications. Chemical Reviews, 114(21), 11060–11082. https://doi.org/10.1021/CR300162P/ASSET/IMAGES/LARGE/CR-2012-00162P_0004.JPEG
-
Su, Y. H., Shih, C. Y., Su, C. H., Chu, I. L., Hsieh, C. T., & Teng, H. (2022). Dielectric gel electrolytes for safe charge storage from −20 to 80°C by double-layer capacitors. Journal of the Taiwan Institute of Chemical Engineers, 134, 104309. https://doi.org/10.1016/J.JTICE.2022.104309
-
Teng, P., Song, G., Cao, Y., Meng, Q., Sun, F., Zhong, N., & Hu, J. (2025). Efficient 1,3–Propanediol production from lignocellulosic hydrolysate via g-C3N4–L. reuteri lighting-driven biohybrid system. Bioresource Technology, 437, 133137. https://doi.org/10.1016/J.BIORTEC