

Volume 1 (2020) 1-6
Synthesis of Silver/Zeolite/LDH Nanocomposite for Simultaneous Removal of Chemical and Pathogenic Bacteria
Siti Nabihan Ishaka, Nik Ahmad Nizam Nik Maleka,b*, Mustaffa Shamsuddinc, Juan Matminc and Gopinathan Sankard
aDepartment of Biosciences, Faculty of Science, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
bCentre for Sustainable Nanomaterials (CSNano), Ibnu Sina Institute for Scientific and Industrial Research (ISI-ISIR), Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
cDepartment of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
dDepartment of Chemistry, Materials Chemistry Section, University College London, United Kingdom
*Corresponding author: niknizam@utm.my
Abstract
Various technologies have been widely developed to provide ideal and safe water. However, it is difficult to simultaneously remove chemical and biological contaminants with low energy consumption and environmentally-friendly way. This research was aimed to synthesize and characterize a silver-zeolite/layered double hydroxide (LDH) nanocomposite as a versatile adsorbent. The synthesized zeolite A, silver-loaded zeolite (Ag-Zeo), LDH, Zeo-LDH and AgZeo-LDH were characterized using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM) and energy dispersive X-ray (EDX), confirming the formation of silver-zeolite/LDH nanocomposite (AgZeo-LDH) where the LDH nanoparticles formed on the Ag-Zeo surfaces. The nanocomposite AgZeo-LDH showed a significant capability in removing both cationic (ammonium) and anionic (phosphate) contaminants in the water as well as antibacterial activity against Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive).
Keywords: Silver; zeolite; layered double hydroxide; nanocomposite; removal; antibacterial
Introduction
Over the past decades, water treatments have received considerable amount of attention because of the importance in providing clean water to humans (Keiser et al., 2019). The accumulation of chemical and biological contaminants in groundwater can cause serious health problems if polluted water is used for daily consumption. Hence, the removal of these contaminants in water bodies is extremely important.
In recent years, one of the proposed approaches to treat contaminants in the water is by using zeolite and layered double hydroxide (LDH) as adsorbents. For example, Bezerra et al. (2019) managed to effectively remove cationic and anionic compounds in petroleum production waste by coating zeolite with LDH. Recently, the zeolite-LDH composite provides greater efficiency to remove toxic compounds such as cadmium (Zhang et al., 2020), toluene (Li et al., 2018), reactive orange six (Belsivo et al., 2020), crystal violet, and methylene blue (Silva et al., 2018). Furthermore, other studies have shown that zeolite is capable of enhancing silver release under a long period to effectively inhibit bacterial growth (Qing et al., 2020; Sanchez et al., 2017; Hanim et al., 2016). Despite the superior qualities of zeolite, LDH, and Ag, there are no studies that synthesize zeolite, LDH and silver in a single composite.
Therefore, this research aims to create a nanocomposite of silver-loaded zeolite with LDH (AgZeo-LDH) to remove cationic and anionic contaminants, and pathogenic bacteria in water media. This study examined the different capabilities of the nanocomposite in eliminating pollutants in the water media: zeolite has high cation exchange capacity (Indarto et al., 2019), LDH has anion exchange capacity (Matusik and Rybka, 2019) and silver has powerful antibacterial activity (Sadoon et al., 2020). Thus, by combining these three materials, the nanocomposite could be used as an economical water filtration material to clean up polluted waters.
Materials and methods
The synthesis of zeolite A and Ag-modified zeolite has been reported previously (Ishak et al., 2020). Based on the study, 1.0 g Ag-Zeo was added with 50 mL of 0.3 M Mg(NO3)2 and 50 mL of 0.1 M Al(NO3)3 in a Teflon bottle. The mixture was stirred at 80°C for 4 h. The pH was maintained at pH 11 by adding 1.0 M Na2CO3 and 1.0 M NaOH. Finally, the resulting solid was washed with distilled water and dried at 60°C in an oven overnight. A similar technique was applied in preparing Zeo-LDH using raw zeolite A.
The synthesis of Mg/Al LDH was carried out following the co-precipitation method in which Mg(NO3)2 and Al(NO3)3 were dissolved in 200 mL of distilled water in the ratio of 3:1 (Mg:Al). The pH was then adjusted to pH 11 using 1M NaOH and 1 M Na2CO3. After that, the mixture underwent hydrothermal treatment at 150°C for 24 h. The generated Mg/Al LDH (LDH) was washed using distilled water and dried at 60°C in an oven overnight.
Each sample was characterized using X-ray diffraction method on a Bruker AXS GmbH (German) diffractometer. Field emission scanning electron microscopy (FESEM) (Hitachi SU8020) analysis was conducted to examine the morphology and energy dispersive X-ray (EDX) for elemental analysis of the samples.
The removal analysis of the samples for NH4+ and PO43- was performed in 40 mL of (NH4)3PO4 100 mg/L with 0.1 g of each material and shaken at 200 rpm for 2 h. The procedure was repeated for zeolite A, LDH and Zeo-LDH. The samples were then filtered and analysed using Macherey-Nagel Nanocolor standard test ammonium (REF 91805) and phosphate (REF 91877).
The antibacterial activity of the samples was studied against Gram-negative bacteria Escherichia coli (ATCC 11229) and Gram-positive bacteria Staphylococcus aureus (ATCC 6538) that were purchased from American Type Culture Collection (ATCC). The antibacterial assay was performed qualitatively using disc diffusion technique (DDT) as stated in previous paper (Ishak et al., 2020).
Results and discussion
Figure 1 shows X-ray diffractogram of the synthesized zeolite A, Ag-Zeo, LDH, Zeo-LDH, and AgZeo-LDH. The diffractogram of zeolite A presented sharp and narrow peaks belonging to a simple cubic crystalline system (Iqbal et al., 2019; Wang et al., 2020). Despite the presence of Ag+ as confirmed using the EDX analysis (Table 1), the unaltered diffractogram suggested that the crystalline structure did not change after Ag+ loading (Salim et al., 2006). The loading of Ag+ in the zeolite was indicated by FESEM images (Figure 2). After the loading, the smooth surfaces of zeolite A were covered by very fine particles, presumably Ag+.
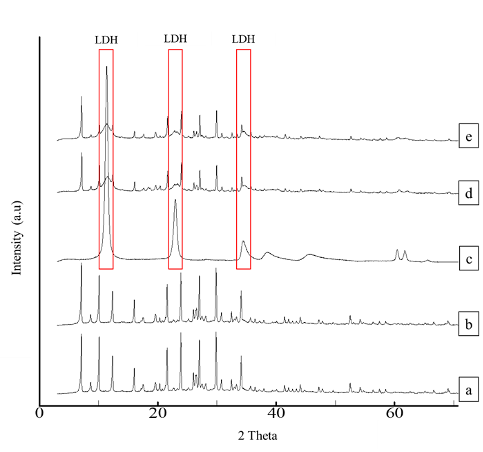
Figure 1 X-ray diffraction pattern of synthesized (a) zeolite A, (b) Ag-Zeo, (c) LDH, (d) Zeo-LDH and (e) AgZeo-LDH.
Referring to Figure 1, the diffractograms of Zeo-LDH and AgZeo-LDH showed that the samples contained LDH and zeolite A as proven by the broad and intense peaks of LDH phase at 2θ= 11.32°, 22.92°, and 34.28°, together with sharp and narrow peaks of zeolite A (Silva et al., 2018; Bezerra et al., 2019; Belsivo et al., 2020). LDH was observed as a nano-sized (130 nm) homogenous layer (Figure 2C). For its composite counterparts, FESEM images showed that the edges of the cubic crystal of Zeo-LDH (Figure 2D) were more defined compared to AgZeo-LDH (Figure 2E). Consequently, in the presence of Ag+, the morphology of the composite was slightly altered without a defined format, but they were totally covered with LDH crystals. The comparison of AgZeo-LDH and Zeo-LDH could be observed clearly in EDX data (Table 1) in which the presence of Ag+ was detected in AgZeo-LDH but not in Zeo-LDH, confirming the formation of the nanocomposite.
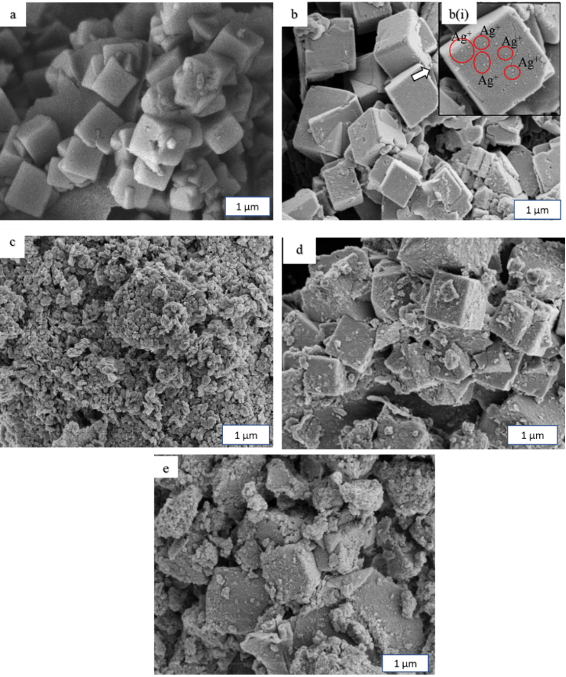
Figure 2 FESEM images of (a) Zeolite A, (b) Ag-Zeo (c) LDH, (d) Zeo-LDH and (e) AgZeo-LDH
Table 1: Elemental analysis of the samples from EDX
|
Elements |
Ag-zeo |
LDH |
Zeo-LDH |
AgZeo-LDH |
|
Si |
14.0 ± 1.44 |
0.0 ± 0.0 |
9.6 ± 1.75 |
6.6 ± 1.66 |
|
Al |
13.2 ± 0.85 |
8.4 ± 3.12 |
11.2 ± 3.39 |
9.6 ± 1.85 |
|
Na |
9.7 ± 1.21 |
0.0 ± 0.0 |
6.8 ± 2.29 |
3.2 ± 1.57 |
|
Mg |
0.0 ± 0.0 |
20.8 ± 6.7 |
2.5 ± 2.24 |
9.7 ± 1.46 |
|
O |
44.3 ±2.62 |
55.6 ± 2.20 |
47.1 ± 7.71 |
50.9 ± 8.77 |
|
C |
16.0 ± 4.48 |
15.2 ± 11.72 |
22.7 ± 16.95 |
17.7 ± 14.54 |
|
S |
0.2 ± 0.06 |
0.0 ± 0.0 |
0.2 ± 0.06 |
0.3 ± 0.0 |
|
Ag |
2.4 ± 1.07 |
0.0 ± 0.0 |
0.0 ± 0.0 |
1.9 ± 0.25 |
In the nanocomposite formation, the desilication of Ag-Zeo in a strong alkaline condition during the nanocomposite formation might produce spaces to be filled with Al from the Al(NO3) sources. The metastable state of Al centre in Ag-Zeo provided an active site for the growth of nanocrystal LDH (Li et al., 2018). In addition to that, Yamada et al. (2006) reported that the assembly of Mg2+ and Al3+ to form LDH was contributed by a high cation-exchange capacity of zeolite A. The discharge of Na from Ag-Zeo allowed Mg2+ from Mg(NO3)2 to refill it, forcing the growth of LDH on the surface of cubic crystal Ag-Zeo to produce the nanocomposite AgZeo-LDH.
In the nanocomposite formation, the desilication of Ag-Zeo in a strong alkaline condition during the nanocomposite formation might produce spaces to be filled with Al from the Al(NO3) sources. The metastable state of Al centre in Ag-Zeo provided an active site for the growth of nanocrystal LDH (Li et al., 2018). In addition to that, Yamada et al. (2006) reported that the assembly of Mg2+ and Al3+ to form LDH was contributed by a high cation-exchange capacity of zeolite A. The discharge of Na from Ag-Zeo allowed Mg2+ from Mg(NO3)2 to refill it, forcing the growth of LDH on the surface of cubic crystal Ag-Zeo to produce the nanocomposite AgZeo-LDH.
The adsorption or removal capacities of AgZeo-LDH nanocomposite against NH4+ cation and PO43- anion in aqueous solution are given in Figure 3. A comparison was made between zeolite A, LDH and Zeo-LDH nanocomposite. The figure shows that raw zeolite can only adsorb NH4+ cation while raw LDH can only adsorb PO43- anion. This result is as expected because the structure of the zeolite is built by the negatively charged aluminosilicate framework that only attracts positive ions or cationic compounds (Kobayashi et al., 2020). For LDH, which is also known as anionic clays, it is composed of layers of Mg-Al hydroxides that can only adsorb anionic compounds (Liu et al., 2020). However, when the LDH was formed on the zeolite surfaces, the Zeo-LDH and AgZeo-LDH nanocomposites showed adsorption capability for both cationic (NH4+) and anionic (PO43-) compounds in the aqueous solution. This was due to the combination of zeolite and LDH that had negative and positive charges, respectively in the nanocomposite. The highest adsorption by AgZeo-LDH nanocomposite was due to the high surface area in the presence of silver ions inside the zeolite framework. The bigger Ag+ ions compared to alkaline cations such as Na+ and K+ ions could expand the pores of zeolite and increase the surface area of the nanocomposite (Chen et al., 2017).
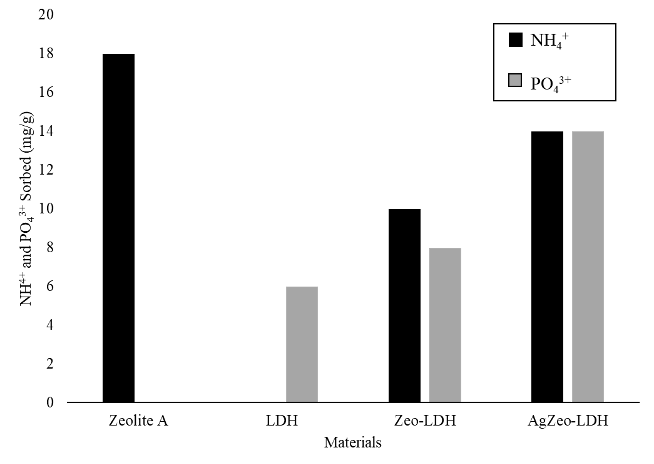
Figure 3: The removal of NH4+ and PO43+ using different materials in 50 mg/L of (NH4)3PO4
In the present study, disc diffusion technique was employed to examine the qualitative antibacterial activity of zeolite A, LDH, Zeo-LDH, and AgZeo-LDH against two types of bacteria, E. coli (Gram negative) and S. aureus (Gram positive). From all the materials studied, only nanocomposite AgZeo-LDH exhibited antibacterial property as evidenced by the presence of inhibition zones (Figure 4). This inhibition activity was caused by the action of Ag+ ions released by the material (Tan et al., 2018), assuming that the antibacterial property of Ag+ still manifested even after the modification with zeolite A and LDH. The proposed mechanism of Ag+ is potentially caused by the penetration of Ag+ through the bacterial membrane, allowing them to enter the cell, disrupt the metabolism of bacteria, cause the production of reactive oxygen species (ROS), induce protein misfolding and silver-thiol complexation, and eventually, cause cell death (Ishida, 2018; Long et al., 2017; Li et al., 2016). Based on the antibacterial property of AgZeo-LDH against both Gram-positive and Gram-negative bacteria, this nanocomposite has the potential to inhibit the growth of a wide spectrum of bacteria.
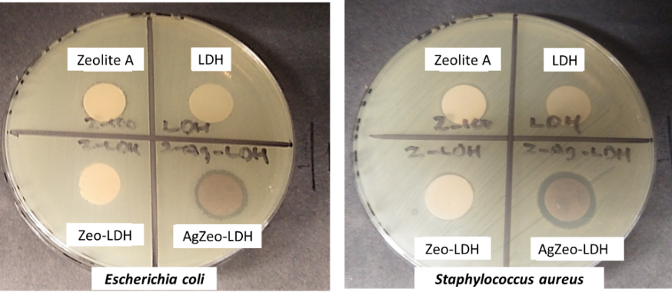
Figure 4: Disc diffusion technique for antibacterial activity of the samples against Escherichia coli (left) and Staphylococcus aureus (right)
Conclusion
The AgZeo-LDH nanocomposite has been successfully created through the addition of AgZeo during the synthesis of Mg/Al LDH as confirmed by XRD, FESEM and EDX. The nanocomposite has the capability to simultaneously remove cationic (NH4+) and anionic (PO43+) contaminants, as well as to inhibit the growth of Gram-positive and Gram-negative bacteria. Therefore, the nanocomposite could be a potential versatile material to eliminate various pollutants as well as bacteria in the water.
Acknowledgement
The authors are grateful to Universiti Teknologi Malaysia, UTM, Skudai for providing a scholarship to Siti Nabihan Ishak under UTM Zamalah scholarship scheme and this project is funded under Kurita Water and Environment Foundation (KWEF) research project (Vot No: 4B410) and UTMShine (Flagship) scheme (Vot No: 03G81).
References
Belviso, C., Piancastelli, A., Sturini, M., & Belviso, S. (2020). Synthesis of composite zeolite-layered double hydroxides using ultrasonic neutralized red mud. Microporous and Mesoporous Materials, 299, 110108.
Bezerra, B., Bieseki, L., da Silva, D., & Pergher, S. (2019). Development of a zeolite A/LDH composite for simultaneous cation and anion removal. Materials, 12(4), 661.
Chen, S., Popovich, J., Iannuzo, N., Haydel, S. E., & Seo, D.-K. (2017). Silver-ion-exchanged nanostructured zeolite X as antibacterial agent with superior ion release kinetics and efficacy against Methicillin-resistant Staphylococcus aureus. Applied Materials & Interfaces, 9(45), 39271–39282.
Hanim, S. A. M., Malek, N. A. N. N., & Ibrahim, Z. (2016). Amine-functionalized, silver-exchanged zeolite NaY: Preparation, characterization and antibacterial activity. Applied Surface Science, 360, 121–130.
Indarto, A., Putra, I. A., Riyano, Noersalim, S., Hartanto, Y., & Handojo, L. (2019). Zeolites as adsorbent materials for decolorization of crude terpineol. IOP Conference Series: Materials Science and Engineering, 599, 012021.
Iqbal, A., Sattar, H., Haider, R., & Munir, S. (2019). Synthesis and characterization of pure phase zeolite 4A from coal fly ash. Journal of Cleaner Production, 219, 258-267.
Ishak, S. N., Malek, N. A. N. N., Yusop, Z., Williams, C. D., Suhartono, S., & Syafiuddin, A. (2020). Evaluation of phase transformation behaviors of zeolite and antibacterial properties against Gram‐positive and ‐negative bacteria. Journal Chinese Chemical Society, In press.
Ishida T. (2018). Antibacterial mechanism of Ag+ ions for bacteriolyses of bacterial cell walls via peptidoglycan autolysins, and DNA damages. MOJ Toxicolology, 4(5), 345–350.
Keiser, D. A. & Shapiro, J. S. (2019). Consequences of the clean water act and the demand for water quality. The Quarterly Journal of Economics, 134(1), 349-396.
Kobayashi, Y., Ogata, F., Nakamura, T., & Kawasaki, N. (2020). Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. Journal of Environmental Chemical Engineering, 8, 103687.
Li, H., Gao, Y., Li, C., Ma, G., Shang, Y., & Sun, Y. (2016). A comparative study of the antibacterial mechanisms of silver ion and silver nanoparticles by Fourier transform infrared spectroscopy. Vibrational Spectroscopy, 85, 112–121.
Li, R., Xue, T., Bingre, R., Gao, Y., Louis, B., & Wang, Q. (2018). Microporous Zeolite@ Vertically Aligned Mg–Al layered double hydroxide core@ shell structures with improved hydrophobicity and toluene adsorption capacity under wet conditions. ACS Applied Materials & Interfaces, 10(41), 34834-34839.
Liu, T., Chen, Y., Yu, Q., Fan, J., & Brouwers, H. J. H. (2020). Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Construction and Building Materials, 250, 118865.
Long, Y.-M., Hu, L.-G., Yan, X.-T., Zhao, X.-C., Zhou, Q.-F., Cai, Y., & Jiang, G.-B. (2017). Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. International Journal of Nanomedicine, 12, 3193–3206.
Matusik, J., & Rybka, K. (2019). Removal of chromates and sulphates by Mg/Fe LDH and heterostructured LDH/halloysite materials: Efficiency, selectivity, and stability of adsorbents in single-and multi-element systems. Materials, 12(9), 1373.
Qing, Y., Li, K., Li, D., & Qin, Y. (2020). Antibacterial effects of silver incorporated zeolite coatings on 3D printed porous stainless steels. Materials Science and Engineering: C, 108.
Sadoon, A. A., Khadka, P., Freeland, J., Gundampati, R. K., Manso, R. H., Ruiz, M. (2020). Silver ions caused faster diffusive dynamics of histone-like Nucleoid-Structuring Proteins in Live Bacteria. Applied and Environmental Microbiology, 2020; 86 (6)
Salim, M. M., & Malek, N. A. N. N. (2016). Characterization and antibacterial activity of silver exchanged regenerated NaY zeolite from surfactant-modified NaY zeolite. Materials Science and Engineering: C, 59, 70–77.
Sánchez, M. J., Mauricio, J. E., Paredes, A. R., Gamero, P., & Cortés, D. (2017). Antimicrobial properties of ZSM-5 type zeolite functionalized with silver. Materials Letters, 191, 65-68.
Silva, L. N. D., Moraes, D. dos S., Santos, S. C. A., & Corrêa, J. A. M. (2018). Joint synthesis of zeolite A-LDH from mineral industry waste. Applied Clay Science, 161, 163–168
Tan, G. Z., Orndorff, P. E., & Shirwaiker, R. A. (2018). The ion delivery manner influences the antimicrobial efficacy of silver oligodynamic iontophoresis. Journal of Medical and Biological Engineering, 39(4), 622–631
Wang, P., Sun, Q., Zhang, Y., & Cao, J. (2019). Synthesis of zeolite 4A from kaolin and its adsorption equilibrium of carbon dioxide. Materials, 12(9), 1536
Zhang, X., Xue, Y., Gao, J., He, C., Ji, Y. and Dou. Y. (2020). Comparison of adsorption mechanisms for cadmium removal by modified zeolites and sands coated with Zn-layered double hydroxides. Chemical Engineering, 380, 122578.

